Tability is a cheatcode for goal-driven teams. Set perfect OKRs with AI, stay focused on the work that matters.
What are Medical Device OKRs?
The Objective and Key Results (OKR) framework is a simple goal-setting methodology that was introduced at Intel by Andy Grove in the 70s. It became popular after John Doerr introduced it to Google in the 90s, and it's now used by teams of all sizes to set and track ambitious goals at scale.
Writing good OKRs can be hard, especially if it's your first time doing it. You'll need to center the focus of your plans around outcomes instead of projects.
We understand that setting OKRs can be challenging, so we have prepared a set of examples tailored for Medical Device. Take a peek at the templates below to find inspiration and kickstart your goal-setting process.
If you want to learn more about the framework, you can read our OKR guide online.
The best tools for writing perfect Medical Device OKRs
Here are 2 tools that can help you draft your OKRs in no time.
Tability AI: to generate OKRs based on a prompt
Tability AI allows you to describe your goals in a prompt, and generate a fully editable OKR template in seconds.
- 1. Create a Tability account
- 2. Click on the Generate goals using AI
- 3. Describe your goals in a prompt
- 4. Get your fully editable OKR template
- 5. Publish to start tracking progress and get automated OKR dashboards
Watch the video below to see it in action 👇
Tability Feedback: to improve existing OKRs
You can use Tability's AI feedback to improve your OKRs if you already have existing goals.
- 1. Create your Tability account
- 2. Add your existing OKRs (you can import them from a spreadsheet)
- 3. Click on Generate analysis
- 4. Review the suggestions and decide to accept or dismiss them
- 5. Publish to start tracking progress and get automated OKR dashboards
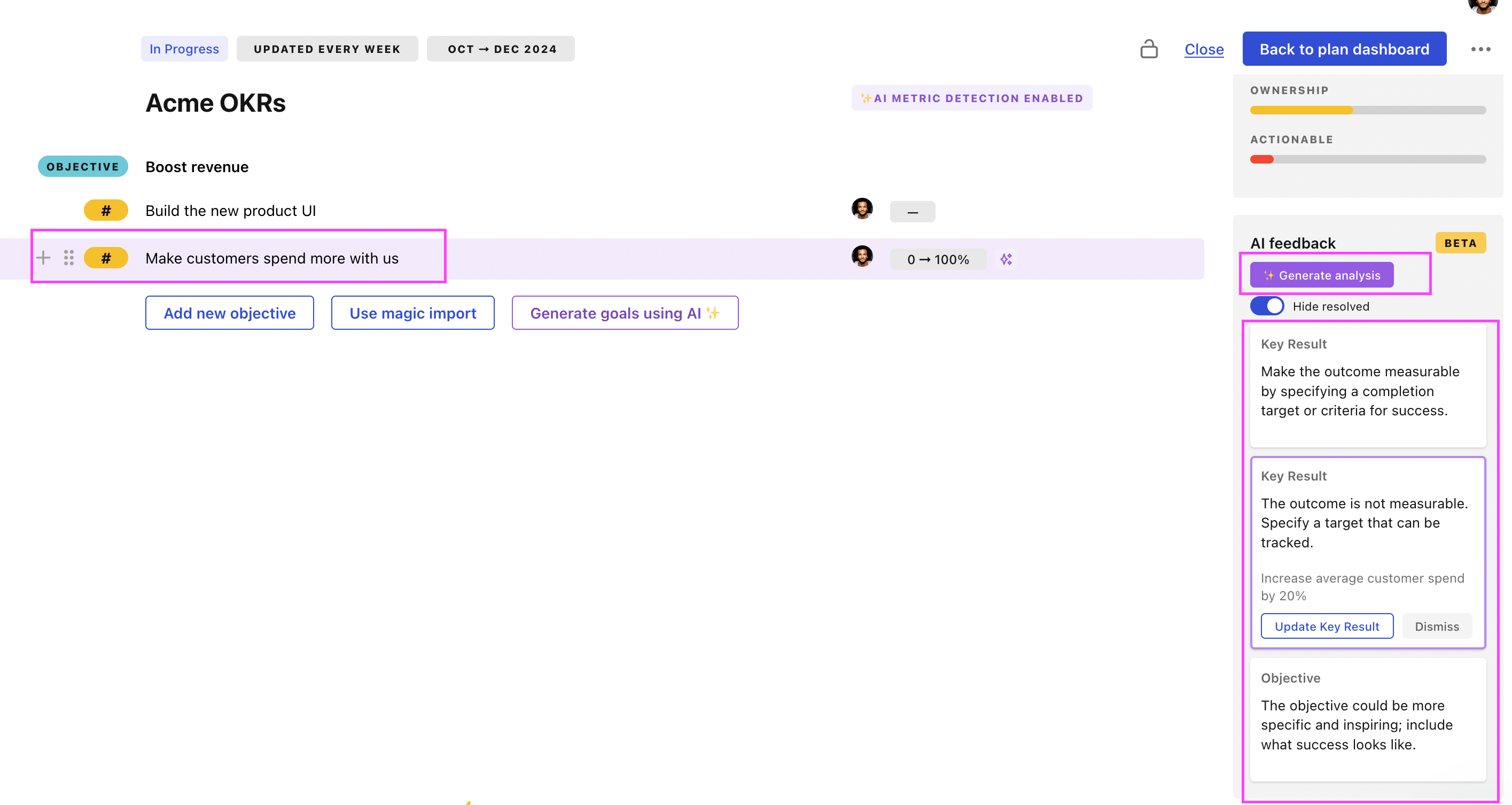
Tability will scan your OKRs and offer different suggestions to improve them. This can range from a small rewrite of a statement to make it clearer to a complete rewrite of the entire OKR.
Medical Device OKRs examples
We've added many examples of Medical Device Objectives and Key Results, but we did not stop there. Understanding the difference between OKRs and projects is important, so we also added examples of strategic initiatives that relate to the OKRs.
Hope you'll find this helpful!
OKRs to successfully patent a new medical device
ObjectiveSuccessfully patent a new medical device
KRComplete comprehensive patent application, including claims and drawings, by Week 8
Draft claims and outline for patent application by Week 3
Finalise and submit patent application by Week 8
Create required drawings for application by Week 5
KRSubmit patent application to USPTO and receive confirmation of receipt by Week 12
Prepare and finalize patent application details
Submit final application to USPTO
Secure confirmation of receipt from USPTO
KRFinalize device design and conduct proof-of-concept tests by Week 4
Execute concept tests and compile results
Complete the final adjustments to device design
Set up a comprehensive schedule for concept tests
OKRs to secure FDA approval for the capital equipment medical device
ObjectiveSecure FDA approval for the capital equipment medical device
KRComplete and submit comprehensive FDA application by week 4
Thoroughly fill out the FDA application form accurately and comprehensively
Gather all necessary documentation and information for FDA application
Submit the completed FDA application before the deadline in week 4
KRObtain confirmation of FDA approval by end of the quarter
Regularly follow-up with FDA regarding application status
Prepare and submit all necessary documentation for FDA approval
Ensure all response deadlines to FDA inquiries are met
KRPass all necessary FDA inspections successfully with zero citations
Implement strict internal quality control measures
Regularly inspect and document all processes for compliance
Review and comprehend all FDA guidelines and expectations
OKRs to file patent for medical device and enhance prototype functionality
ObjectiveFile patent for medical device and enhance prototype functionality
KRThoroughly research patents in the related field to ensure uniqueness by end of Week 4
Identify relevant fields for patent search
Analyze findings for potential uniqueness conflicts
Conduct comprehensive patent search through online databases
KRImplement three major improvements in the prototype based on testing feedback by Week 12
KRComplete drafting a unique and detailed patent application by Week 8
Finalize application, including all diagrams and claims
Write a comprehensive description of the invention
Research and analyze existing patents relevant to the invention
Medical Device OKR best practices
Generally speaking, your objectives should be ambitious yet achievable, and your key results should be measurable and time-bound (using the SMART framework can be helpful). It is also recommended to list strategic initiatives under your key results, as it'll help you avoid the common mistake of listing projects in your KRs.
Here are a couple of best practices extracted from our OKR implementation guide 👇
Tip #1: Limit the number of key results
The #1 role of OKRs is to help you and your team focus on what really matters. Business-as-usual activities will still be happening, but you do not need to track your entire roadmap in the OKRs.
We recommend having 3-4 objectives, and 3-4 key results per objective. A platform like Tability can run audits on your data to help you identify the plans that have too many goals.
Tip #2: Commit to weekly OKR check-ins
Don't fall into the set-and-forget trap. It is important to adopt a weekly check-in process to get the full value of your OKRs and make your strategy agile – otherwise this is nothing more than a reporting exercise.
Being able to see trends for your key results will also keep yourself honest.
Tip #3: No more than 2 yellow statuses in a row
Yes, this is another tip for goal-tracking instead of goal-setting (but you'll get plenty of OKR examples above). But, once you have your goals defined, it will be your ability to keep the right sense of urgency that will make the difference.
As a rule of thumb, it's best to avoid having more than 2 yellow/at risk statuses in a row.
Make a call on the 3rd update. You should be either back on track, or off track. This sounds harsh but it's the best way to signal risks early enough to fix things.
Save hours with automated Medical Device OKR dashboards
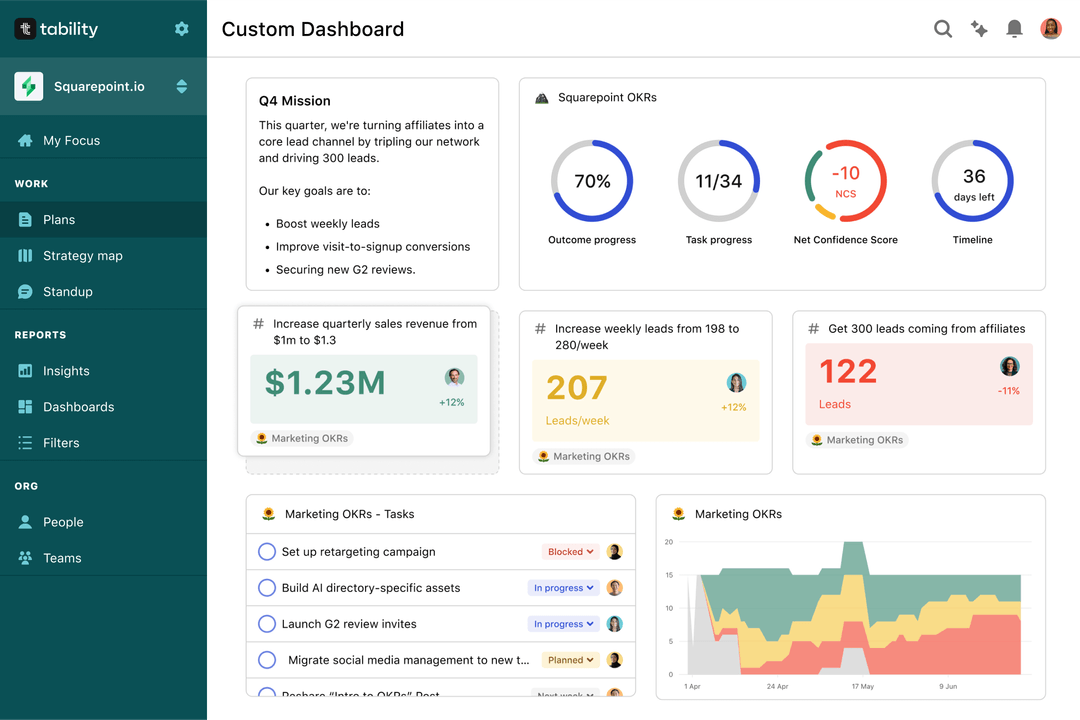
OKRs without regular progress updates are just KPIs. You'll need to update progress on your OKRs every week to get the full benefits from the framework. Reviewing progress periodically has several advantages:
- It brings the goals back to the top of the mind
- It will highlight poorly set OKRs
- It will surface execution risks
- It improves transparency and accountability
Spreadsheets are enough to get started. Then, once you need to scale you can use Tability to save time with automated OKR dashboards, data connectors, and actionable insights.
How to get Tability dashboards:
- 1. Create a Tability account
- 2. Use the importers to add your OKRs (works with any spreadsheet or doc)
- 3. Publish your OKR plan
That's it! Tability will instantly get access to 10+ dashboards to monitor progress, visualise trends, and identify risks early.
More Medical Device OKR templates
We have more templates to help you draft your team goals and OKRs.
OKRs to implement Crowdstrike enterprise endpoint security with new features
OKRs to successfully rebrand two custom studios
OKRs to enhance employee satisfaction with total remuneration
OKRs to establish uninterrupted power supply for all cable and net nodes during blackouts
OKRs to implement new functionality in our product offering
OKRs to implement the new onboarding program to speed up deal closure time